The earliest documentary record of the term ‘chemistry’ is traced to a decree of Emperor Diocletian in 296 AD proscribing chemistry and ordering to burn the Egyptian books on ‘chemeia’ relating to making gold or silver by imitation. Others etymologies of ‘chemistry’ derive the word ‘chemist’ from Egyptian ‘cheme’; the fertile black soil left every year after the flooding of the Nile river. In consideration of the services rendered to humanity, chemistry can be deservedly taken as the Mother of Sciences.
1. Abu Musa Jabir ibn Hayyan

Also known as Gebr or Tusi, Abu Musa Jabir ibn Hayyan was born in Tus, Persia in C.721 and died in Kufa, Iraq in C.815. Primarily regarded as the first practical chemist, he was an ambidextrous scholar and is also known as a physician, astronomer, and pharmacist. As recorded in ‘The Cultural Atlas of Islam’ by Ismail al-Faruqi (New York, 1986, p. 328), ‘In response to Jafar al-Sadik’s wishes, he invented a kind of paper that resisted fire and an ink that could be read at night. He invented an additive which, when applied to an iron surface, inhibited rust and when applied to a textile, would make it water repellent.’
2. Dmitri Mendeleev

Born at Toboslk, Siberia in 1834, DimitriMendeleev, died in St. Petersburg on February 2, 1907. ‘Last but not the least’ applies to him literally as he was the last of the 14 children of Ivan and Mariya Mendeleev. He studied science at St. Petersburg; graduating in 1856 and later on became a professor of Chemistry in the same institution. Known as a ‘Chemist of Genius,’ he researched in many fields including: meteorology, geology, physics, and industrial chemistry. His greatest achievement is devising periodic law and development of the Periodic Table. What the alphabet is to language, the Periodic Table is to chemistry. Anyone having the least touch with chemistry is very well aware of the importance of the Periodic Table. He invented the effective Periodic Table in 1869. Menedeleev’s laws including Mendeleev’s ‘Law of Periodicity’ and Mendeleev’s ‘Law of Octaves’ were named after him.
3. Fredrick Sanger

Nobel Prize Laureates stand prominently among the world-famous scientists as a result of their achievements recognized at the highest level. Fredrick Sanger is one of the four Nobel Laureates who stood out prominently among the Noble Laureates. Fredrick Sanger was born in Rendcombe, England on August 13, 1918. He earned his doctorate from St.John’s College in 1943. For the first time, in 1958, he was awarded the Nobel Prize for discovering the structure of insulin. The second time he was awarded the Nobel Prize in 1980 for his research relating to the sequencing of DNA. This time he shared the prize with Paul Berg and Walter Gilbert.
4. Emil Fischer

Emil Fischer was born in Euskirchen in the Cologne district of Germany on October 9, 1852. According to his autobiography, he was considered too stupid by his father to be a businessman; therefore, he was sent to study chemistry at the University of Bonn. His works included the synthesis of many chemicals, but his greatest achievement was the synthesis of purine. His works included the synthesis of glucose, fructose, and mannose from glycerol. He was awarded the Nobel Prize for Chemistry in 1902.
5. Alfred Nobel

Alfred Nobel was born on October 21, 1883 and died of a stroke on December 10, 1896.
He invented many things like: synthetic rubber, artificial silk, and leather. By 1896 he had 355 patents. His prime invention was the controlled form of trinitroglycerine, an explosive which was invented three years earlier but was not controllable or usable as it exploded during the preparatory stages. AlfredNobel made a paste of the trinitroglycerin with kieselguhr and formed it into rods provided with detonators. He patented this invention in 1867. By his will he gifted 31,225,000 Swedish kroners, equivalent to 250 million U.S. Dollars in 2008. The Nobel Prize was founded in 1895, and since 1901 the Nobel Prize has been awarded every year for achievements in chemistry, physics, medicine, literature, and work done for the cause of peace.
6. Henry Cavendish

Henry Cavendish was born in Nice, France on 1731 and died in London, England on February 24, 1810. He was the eldest son of Lord Charles Cavendish and Lady Anne Grey. He got admission in Peterhouse, Cambridge in 1749 but did not get a degree and left it after three years. He was a chemist and a millionaire by inheritance. He discovered the composition of air showing that air was a compound and not an element. He also invented nitric acid. He measured the density and mass of the Earth by the famous Cavendish Experiment. His works include the discovery of the Columbus Law. His academic report presented in 1766 ‘Three Papers Containing Experiments on Factitious Air’ revealed the formation of the ‘inflammable air’ hydrogen by the action of acids on metals.
7. R. B. Woodward

Robert Burns Woodward was born on April 10, 1917 and died on July 8, 1979. He was the only child of Arthur Woodward and Margaret Burns. He got his doctorate in 1937 and was associated with Harvard University afterwards. He held more than 20 honorary degrees. He was awarded the Nobel Prize for Chemistry in 1965 for his work on Organic Synthesis. He occupies one of the foremost positions among organic chemists. A few of his awards include the following but do not exclude many others: Davy Medal of Royal Society 1959, Roger Adams Medal; American Chemical Society 1961, Pius XI Gold Medal; Pontifical Academy of Sciences 1969, and National Medal of Science, United States of America 1964.
8. Antoine Lavoisier

Antoine Lavoisier was born on August 26, 1743 in Paris, France and died on May 8, 1794. He is best known for proving the Law of Conservation of Mass, which is a fundamental principle of physics and states that ‘Matter can neither be created nor destroyed in an isolated system.’ He was the first to state that air was a mixture of oxygen and ‘azote’; now known as ‘nitrogen.’ In 1787 he published ‘Methods of Chemical Nomenclature’ which is regarded the first textbook of modern chemistry. He was beheaded by the anti-monarchists during theFrenchRevolution.
9. G. N. Lewis

Gilbert N. Lewis was born at Waymouth, Massachusetts on October 23, 1875 and died on March 23,1946 at Berkeley, California. He got his doctorate from Harvard University under the direction of Theodore Richards. He is best known for explaining Covalent Bonds, Lewis Structures, Electrochemical theory of acids and bases. He coined the term ‘photon’ for the smallest unit of energy obtained from radiation. His prime achievement was the discovery of the ‘Covalent Bond’ reported through his paper on ‘The Atom and the Molecule’ in 1916.
10. Linus Pauling
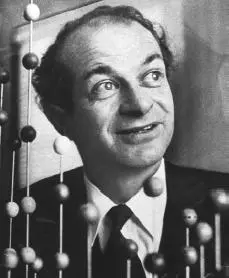
Linus Pauling was born on February 28, 1901 at Portland, Oregon, U.S. and died on August 19, 1994 at the age of 93 in Big Sur, California U.S. His unique and distinguishing honor is that he was the only scientist who won the undivided Nobel Prize twice. He is famous for defining the nature of chemical bonds, the structure of molecules, and for advocating nuclear disarmament. His movement was restricted in the beginning as he was considered pro-Soviet but later on was awarded the Nobel Prize for his persistent, anti-nuclear stand.
Conclusion:
Chemists, among the fraternity of scientists, have been on the forefront of developing the magnanimous sources of energy, be it the explosive power of dynamite or nuclear energy. They have always presented the energy after they have harnessed it, but mostly the decision makers and practical consumers have been the politicians who, as the historical records show, failed to show due diligence and unleashed it against humanity. Discoveries of great chemists are double-edged swords which have been used at the whims of those in power.










June 24, 2014 2:29 pm
Regarding Cavendish:
“air showing that air was a compound and not an element”
This is not quite right. Air is a mixture of various substances, many of which are elements (nitrogen, oxygen, hydrogen) and many of which are compounds (also nitrogen N2, oxygen O2, hydrogen H2 and also water H2O and carbon dioxide CO2). Some of the components of air are both elements and in their elemental form (argon Ar, helium He, and neon, Ne).